STANDARD 9
OVERVIEW
There are two types of data: administrative data and patient reported experience/outcome measures (PROMs and PREMs) and collecting this data helps to improve business operations and patient care. The following is recommended to implement measurement of quality care at your clinic:
- Collect data electronically using an Electronic Medical Record (EMR) system with effective extraction
- Work towards achieving four measurement goals:
- Define the cohort of the OA patients at your centre. This may be done by adding a specific label (e.g. ‘OA’) or creating a ‘panel’
- Collect PROMs every 12 months at a minimum to track the individual’s OA symptom progression (EQ5D-5L and the AOAPF)
- Collect PREMs to ensure the individual’s perspective is informing the delivery of care (AOAEM)
- Care plan created using the hierarchy of treatments for each patient (or follow care plan if supporting provider)
- Assign clear roles in your team for responsibilities with tracking, analyzing, interpreting, and actioning measurement results to continuously drive the improvement of the quality of your care
The BJH SCN and ABJHI are committed to making big improvements in Alberta’s conservative OA care. It will take time to build many trusting partnerships with multi-disciplinary providers and with individuals. If your organization would like to share your data with ABJHI, or partner with ABJHI to strengthen your data collection, please do not hesitate to get in touch: info@albertaboneandjoint.com
INTRODUCTION
Alberta has a proud history of demonstrating successful high-quality healthcare using measurement of outcomes, particularly in bone and joint healthcare. We define quality care with six dimensions: acceptability, accessibility, appropriateness, effectiveness, efficiency and safety.
Bringing measurement into your local setting can help you improve business operations as well as demonstrate that the care you are delivering is of high quality and successful in supporting individuals with OA.
Types of Data
There are two types of data that can be collected:
- Administrative data and
- ‘Patient reported’ data provided by the individual with OA
Administrative data is the responsibility of the provider and can include anything to do with the workflow at the local centre. Collecting administrative data helps improve business operations to offer efficient and accessible services for the individual with OA.
‘Patient reported’ data consists of two types:
- Patient Reported Outcome Measures (PROMs)
- Patient Reported Experience Measures (PREMs)
It is important to gather both PROMs and PREMs to properly evaluate the effectiveness, acceptability and appropriateness of the care delivered. See below for guidance on which PROMs and PREMs to use for conservative management of OA.
MEASURING OUTCOMES
- Collect data electronically using an Electronic Medical Record (EMR) system with effective extraction.
- Work towards achieving four measurement goals:
- Monitoring your OA patients as a cohort at your centre. This may be done by adding a specific label (e.g. ‘OA’) or creating a ‘panel’
- Collect PROMs every 12 months at a minimum to track the individual’s OA symptom progression (unless discharged – Standard 3)
- For the purposes of conservative management of OA EQ5D-5L, the Subjective OA Performance Score (SOAPS) are recommended
- The Patient Specific Functional Scale (PSFS) is also a useful tool for attaching outcomes to goals the individual has set
- The Health Resources Matrix provides guidance on which PROM/PREM is appropriate for which Standard
- Collect PREMs to ensure the individual’s perspective is informing the delivery of care
- For the purposes of conservative management of OA: the Alberta OA Experience Measure (AOAEM) is recommended and
- The Health Resources Matrix provides guidance on which PROM/PREM is appropriate for which Standard
- Care plan created using Conservative OA Treatments for each patient (or follow care plan if supporting provider)
- Assign clear roles in your team for responsibilities with tracking, analyzing, interpreting, and actioning measurement results to continuously drive the improvement of the quality of your care
- If your organization would like assistance in this area, you can contact Alberta Bone and Joint Health Institute (ABJHI) for information on services that are available: info@albertaboneandjoint.com
HOW TO START
- Are you collecting Electronically or manually?
- Are you using an Electronic Medical Record (EMR) system?
- Consider collecting data electronically to strengthen data tracking and data preservation
- Consider using an EMR that provides you with the flexibility to adapt your data collection to your needs
- What does the evidence tell you is a reasonable frequency to collect data?
- How can you balance this with the administrative duties of your business?
- Standard 3 provides some guidance on reasonable frequency for checking on an individuals’ progress with specific types of treatments
- Which team members are responsible for managing the data?
- Which team members will review the data?
- Consider having one team member for managing data, and one for reviewing
- Planning ahead about data collection methods and frequencies will make these roles more straightforward
- Before you make any changes to your care: what is the current quality of your care?
- Change cannot be measured if there is nothing to compare it to
- Gather a baseline before making any changes to the care your team delivers
- How will your full team know what the data says about the care they are delivering?
- How will your team understand the importance of the changes they need to make?
- How will you celebrate your team for the changes they do make?
- Consider using simple graphs to highlight changes and celebrate success
- Which team members will be responsible for investigating your data?
- How will your team make decisions about what changes to make to your service delivery to improve your data?
- How will you hold each other accountable to track those changes?
- Form a quality team that meets regularly
- Review the data together and understand what it says about the quality of your service delivery
- Work together to understand and investigate problems
- Carefully plan changes including understanding which data should shift if the change can be identified as successful
RESEARCH VS QUALITY IMPROVEMENT
A Project Ethics Community Consensus Initiative (ARECCI) program is hosted by Alberta Innovates. It was established to assist with the planning for ethical risks for projects that are not research projects. These projects might include program evaluations, quality improvement, health innovations or knowledge translation. All of these projects involve people and their information and therefore careful planning is required to protect that information. ARECCI provides support for this planning by providing decision support tools, training opportunities and ethics consultants.
ARECCI has all their tools and additional information available here. In particular, the ARECCI Screening Tool is useful for determining if your project is a research project or not. The Screening Tool asks questions to help you think through the purpose of your project.
Quality Improvement vs. Research Projects
| Quality Improvement | Research | |
|---|---|---|
| Purpose | Use existing knowledge to improve local care | Discover new, generalizable knowledge |
| Strategy | Multiple small sequential observable tests; interventions, adapted based on learnings | Usually one large, well-controlled study; interventions planned in advance |
| Ethics Review | Performed according to local policies | Performed by a research ethics board |
| Sample Size | Focus on gathering enough information to achieve a reliable measurement; project continues until aim is achieved and may involve ongoing measurement to ensure change is sustained | Sample size calculation with goal of adequate power to detect a meaningful difference; study ends when enrolment met |
| Analysis | Occurs in an ongoing manner throughout multiple tests of change; often utilizes run charts and control charts | Occurs after data collection complete or at a defined interim analysis; often utilizes hypothesis testing |
| Dissemination | Findings shared locally; may be shared broadly for purpose of sharing learning from local efforts | Findings shared broadly for purpose of increasing knowledge |
Quality Improvement vs. Research Data
| Quality Improvement | Research | |
|---|---|---|
| Aim | To bring new knowledge into daily practice | To discover new knowledge |
| Tests | Many sequential, observable tests | One large blind test |
| Bias | Accept consistent bias | Design to eliminate bias |
| Sample Size | Gather “just enough” data to learn & complete another cycle | Gather as much data as possible, “just in case” |
| Measuring Improvement | Run charts, Shewhart control charts | Hypothesis, stat tests (t-test, F-test, chi square), p-values |
| Confidentiality | Data used only by those involved | Subjects’ identities protected |
INTRODUCTION
Bringing measurement into your local setting can help you improve business operations as well as demonstrate that the care you are delivering is of high quality and successful in supporting individuals with OA.
Types of Data
There are two types of data that can be collected:
- Administrative data and
- ‘Patient reported’ data provided by the individual with OA
Administrative data is the responsibility of the provider and can include anything to do with the workflow at the local centre. Collecting administrative data helps improve business operations to offer efficient and accessible services for the individual with OA.
‘Patient reported’ data consists of two types:
- Patient Reported Outcome Measures (PROMs)
- Patient Reported Experience Measures (PREMs)
It is important to gather both PROMs and PREMs to properly evaluate the effectiveness, acceptability and appropriateness of the care delivered. See below for guidance on which PROMs and PREMs to use for conservative management of OA.
MEASURING OUTCOMES
- Collect data electronically using an Electronic Medical Record (EMR) system with effective extraction.
- Work towards achieving four measurement goals:
- Monitoring your OA patients as a cohort at your centre. This may be done by adding a specific label (e.g. ‘OA’) or creating a ‘panel’
- Collect PROMs every 12 months at a minimum to track the individual’s OA symptom progression (unless discharged – Standard 3)
- For the purposes of conservative management of OA EQ5D-5L, the Subjective OA Performance Score (SOAPS) are recommended
- The Patient Specific Functional Scale (PSFS) is also a useful tool for attaching outcomes to goals the individual has set
- The Health Resources Matrix provides guidance on which PROM/PREM is appropriate for which Standard
- Collect PREMs to ensure the individual’s perspective is informing the delivery of care
- For the purposes of conservative management of OA: the Alberta OA Experience Measure (AOAEM) is recommended and
- The Health Resources Matrix provides guidance on which PROM/PREM is appropriate for which Standard
- Care plan created using Conservative OA Treatments for each patient (or follow care plan if supporting provider)
- Assign clear roles in your team for responsibilities with tracking, analyzing, interpreting, and actioning measurement results to continuously drive the improvement of the quality of your care
- If your organization would like assistance in this area, you can contact Alberta Bone and Joint Health Institute (ABJHI) for information on services that are available: info@albertaboneandjoint.com
HOW TO START
- Are you collecting Electronically or manually?
- Are you using an Electronic Medical Record (EMR) system?
- Consider collecting data electronically to strengthen data tracking and data preservation
- Consider using an EMR that provides you with the flexibility to adapt your data collection to your needs
- What does the evidence tell you is a reasonable frequency to collect data?
- How can you balance this with the administrative duties of your business?
- Standard 3 provides some guidance on reasonable frequency for checking on an individuals’ progress with specific types of treatments
- Which team members are responsible for managing the data?
- Which team members will review the data?
- Consider having one team member for managing data, and one for reviewing
- Planning ahead about data collection methods and frequencies will make these roles more straightforward
- Before you make any changes to your care: what is the current quality of your care?
- Change cannot be measured if there is nothing to compare it to
- Gather a baseline before making any changes to the care your team delivers
- How will your full team know what the data says about the care they are delivering?
- How will your team understand the importance of the changes they need to make?
- How will you celebrate your team for the changes they do make?
- Consider using simple graphs to highlight changes and celebrate success
- Which team members will be responsible for investigating your data?
- How will your team make decisions about what changes to make to your service delivery to improve your data?
- How will you hold each other accountable to track those changes?
- Form a quality team that meets regularly
- Review the data together and understand what it says about the quality of your service delivery
- Work together to understand and investigate problems
- Carefully plan changes including understanding which data should shift if the change can be identified as successful
RESEARCH VS QUALITY IMPROVEMENT
A Project Ethics Community Consensus Initiative (ARECCI) program is hosted by Alberta Innovates. It was established to assist with the planning for ethical risks for projects that are not research projects. These projects might include program evaluations, quality improvement, health innovations or knowledge translation. All of these projects involve people and their information and therefore careful planning is required to protect that information. ARECCI provides support for this planning by providing decision support tools, training opportunities and ethics consultants.
ARECCI has all their tools and additional information available here. In particular, the ARECCI Screening Tool is useful for determining if your project is a research project or not. The Screening Tool asks questions to help you think through the purpose of your project.
Quality Improvement vs. Research Projects
| Quality Improvement | Research | |
|---|---|---|
| Purpose | Use existing knowledge to improve local care | Discover new, generalizable knowledge |
| Strategy | Multiple small sequential observable tests; interventions, adapted based on learnings | Usually one large, well-controlled study; interventions planned in advance |
| Ethics Review | Performed according to local policies | Performed by a research ethics board |
| Sample Size | Focus on gathering enough information to achieve a reliable measurement; project continues until aim is achieved and may involve ongoing measurement to ensure change is sustained | Sample size calculation with goal of adequate power to detect a meaningful difference; study ends when enrolment met |
| Analysis | Occurs in an ongoing manner throughout multiple tests of change; often utilizes run charts and control charts | Occurs after data collection complete or at a defined interim analysis; often utilizes hypothesis testing |
| Dissemination | Findings shared locally; may be shared broadly for purpose of sharing learning from local efforts | Findings shared broadly for purpose of increasing knowledge |
Quality Improvement vs. Research Data
| Quality Improvement | Research | |
|---|---|---|
| Aim | To bring new knowledge into daily practice | To discover new knowledge |
| Tests | Many sequential, observable tests | One large blind test |
| Bias | Accept consistent bias | Design to eliminate bias |
| Sample Size | Gather “just enough” data to learn & complete another cycle | Gather as much data as possible, “just in case” |
| Measuring Improvement | Run charts, Shewhart control charts | Hypothesis, stat tests (t-test, F-test, chi square), p-values |
| Confidentiality | Data used only by those involved | Subjects’ identities protected |
How to Start Measuring Outcomes at Your Clinic
Consider the following prompts as your planning develops to incorporate measurement into your clinic practice and your service delivery matures. Measuring the quality of your care will improve business operations and the experiences of your individuals with osteoarthritis (OA).
| Item to Consider | Questions to Ask | Tips |
|---|---|---|
| How you collect your data |
|
|
| The frequency at which you will collect your data |
|
|
| Roles of team members |
|
|
| Establishing a baseline |
|
|
| Communicate results to the team |
|
|
| Using the data to continuously drive quality improvement of care |
|
|
Research vs. QI
A Project Ethics Community Consensus Initiative (ARECCI) program is hosted by Alberta Innovates. It was established to assist with the planning for ethical risks for projects that are not research projects. These projects might include program evaluations, quality improvement, health innovations or knowledge translation. All of these projects involve people and their information and therefore careful planning is required to protect that information. ARECCI provides support for this planning by providing decision support tools, training opportunities and ethics consultants.
ARECCI has all their tools and additional information available here. In particular, the ARECCI Screening Tool is useful for determining if your project is a research project or not. The Screening Tool asks questions to help you think through the purpose of your project.
Quality Improvement vs. Research Projects
| Quality Improvement | Research | |
|---|---|---|
| Purpose | Use existing knowledge to improve local care | Discover new, generalizable knowledge |
| Strategy | Multiple small sequential observable tests; interventions, adapted based on learnings | Usually one large, well-controlled study; interventions planned in advance |
| Ethics Review | Performed according to local policies | Performed by a research ethics board |
| Sample Size | Focus on gathering enough information to achieve a reliable measurement; project continues until aim is achieved and may involve ongoing measurement to ensure change is sustained | Sample size calculation with goal of adequate power to detect a meaningful difference; study ends when enrolment met |
| Analysis | Occurs in an ongoing manner throughout multiple tests of change; often utilizes run charts and control charts | Occurs after data collection complete or at a defined interim analysis; often utilizes hypothesis testing |
| Dissemination | Findings shared locally; may be shared broadly for purpose of sharing learning from local efforts | Findings shared broadly for purpose of increasing knowledge |
Quality Improvement vs. Research Data
| Quality Improvement | Research | |
|---|---|---|
| Aim | To bring new knowledge into daily practice | To discover new knowledge |
| Tests | Many sequential, observable tests | One large blind test |
| Bias | Accept consistent bias | Design to eliminate bias |
| Sample Size | Gather “just enough” data to learn & complete another cycle | Gather as much data as possible, “just in case” |
| Measuring Improvement | Run charts, Shewhart control charts | Hypothesis, stat tests (t-test, F-test, chi square), p-values |
| Confidentiality | Data used only by those involved | Subjects’ identities protected |
Six Dimensions of Quality
The Health Quality Council of Alberta was established under legislature in 2003. They developed the Alberta Quality Matrix for Health which has widespread success, particularly in driving quality improvement in Alberta Health Services’ (AHS’) Strategic Clinical Networks (SCNs).
The Six Dimensions of Quality can be defined as:
| Dimension | Definition |
|---|---|
| Acceptability | Health services are respectful and responsive to user needs, preferences and expectations |
| Accessibility | Health services are obtained in the most suitable setting in a reasonable time and distance |
| Appropriateness | Health services are relevant to user needs and are based on accepted or evidence-based practice |
| Effectiveness | Health services are based on scientific knowledge to achieve desired outcomes |
| Efficiency | Resources are optimally used in achieving desired outcomes |
| Safety | Mitigate risks to avoid unintended or harmful results |
More information on HQCA can be found here: https://www.hqca.ca/
More information on AHS’ SCNs can be found here: https://www.albertahealthservices.ca/scns/scn.aspx
The complete Alberta Quality Matrix for Health can be found here: https://hqca.ca/about-us/our-mandate/the-alberta-quality-matrix-for-health/
Administrative Data
Administrative data includes anything that can be collected about how the clinicians work to deliver the care to the individuals. This likely includes time stamps to document when certain milestones are hit such as:
- The date a referral is received
- The date of a first appointment
- The date of subsequent appointments
- The date a treatment is completed
- The date of discharge
Administrative data can also include charting completed by the clinicians to collect key characteristics about the individuals. It is best if this information is translated out of long form answers into simple check boxes as much as possible to make it easier to extract the information for analysis later.
Administrative data can be collected on paper or electronically in an electronic medical record (EMR) software system. Electronic recording of administrative data exponentially improves the ability to analyze data for quality improvement initiatives.
It is very important to combine administrative data with ‘patient reported’ data from the individual to get a complete picture of the quality of care being delivered.
Shared Decision Making
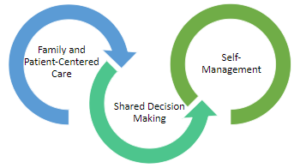
The inter-twining principles that ground the Comprehensive Quality Care Standards for Osteoarthritis of the Hip and Knee.
Shared decision-making (SDM) techniques and processes are emphasized throughout these standards. The use of SDM is essential to achieving ‘family and patient-centric’ care. SDM is evidence-based and proven to improve outcomes for the individual such as satisfaction with and adherence to care plans.
Standard 2 describes how sharing the decision making is important for building a care plan. This toolkit This toolkit provides more detail on the 3-talk model that can be used to practice SDM in everyday practice.
Advanced Pain Management
Opioids are not recommended for routine use to treat osteoarthritis (OA) pain. Opioids have harmful side effects, including:
- Cognitive effects
- Increased risk of falls
- Increase in pain symptoms (opioid hyperalgesia)
- Risk of tolerance that may lead to dose escalation
- Risk of addiction and
- Risk of overdose, particularly if used in combination with other prescribed medications, alcohol, and/or in the presence of other health conditions including sleep apnea.
Prior to prescribing opioids, assess all risks and the medical history of the individual carefully. If the individual with OA requires advanced pain management, the clinicians should aim to prescribe the lowest effective dose, ideally <50mg morphine equivalents per day, and for a short duration. The Canadian Opioid Prescribing Guideline can also be consulted to provide further guidance. A strong opioid prescription plan for the individual includes:
- A clear discussion of the benefits and risks of opioids
- Instructions on when and how to consume the medication
- Instructions on the duration of the prescription
- A plan for frequent follow-up with the prescriber to ensure that the medication is helping for pain and function and not causing adverse side effects
- A naloxone kit with each prescription if prescribing to individuals at risk, such as those who:
- Are receiving a high dose
- Have a complex medical history and
- Have comorbidities
- A clear discussion about how short-term opioid use is meant to support the continued participation in Core Treatments.
The clinician should diligently monitor changes in the individual’s function, adherence to prescribed dose and frequency and any adverse side effects, which may require a treatment modification.
Peripheral Nerve Blocks
The body of evidence for injection treatments for osteoarthritis (OA) is evolving. Many OA clinical guidelines do not provide conclusive recommendations because of this. However, some individuals with OA respond very well to injections and can use them to continue comfortably with their Core Treatments.
Peripheral nerve blocks can be used at the knee to provide pain relief from OA. The geniculate nerve wraps around the knee and provides sensation to the joint. With ultrasound guidance, this nerve can be isolated and local anaesthetic can be infiltrated around the nerve. If ‘freezing’ the nerve results in pain relief for a few hours, this is a good indication that a procedure termed a radiofrequency ablation (‘burning’) of the geniculate nerve will provide more lasting pain relief.
Genicular Nerve Radiofrequency Ablation for Chronic Knee Pain | Spine and Pain Management in Fairfax, VAThe radio-frequency ablation is a simple, 10-minute procedure where a probe is passed through a small needle onto the nerve. Radio-frequency electrical waves are applied to the probe which ablates the nerve making it unable carry signals to the brain to sense pain. When this technique is applied to a knee with OA, six-to-12 months of pain relief is anticipated.
There is a risk of hypoesthesia as an adverse event. Individuals with OA should be observed for stability, perhaps complete a TUG test, before they are discharged from the treatment session.
Intra-articular Injections
The body of evidence for injection treatments for osteoarthritis (OA) is evolving. Many OA clinical guidelines do not provide conclusive recommendations because of this. However, some individuals with OA respond very well to injections and can use them to continue comfortably with their Core Treatments.
Different injections have different purposes, and for some individuals a combination of injections may be appropriate, and different compounds may be injected at different times:
- Steroid (i.e. cortisone) is a potent anti-inflammatory medication. Steroids generally works quickly and generally does not provide lasting benefits compared to hyaluronic acid and/or platelet rich plasma. Steroids provide moderate pain relief and restoration of function; they are also cost-effective. Steroids are conditionally recommended for acute (1-2 weeks) and short term (4-6 weeks) pain relief. Consider repetitive injections if effective, no more often than three-four months.
- Hyaluronic acid (HA) is a compound found in normal, healthy joint fluid. HA is diminished in arthritic knees. HA injections are best in an arthritic joint, i.e. one that is not swollen. HA effects generally are more long lasting compared to steroids, possibly providing benefit for six-twelve months. HA provide improved pain relief and restoration of function compared with placebo.
- Theoretically, to be most effective the HA preparation has to be high molecular weight and highly cross-linked.
- Common OA clinical practice guidelines has been published for use with knees only, however it has been used effectively for hips as well.
- Platelet rich plasma (PRP) is a form of biologic injection that is an emerging therapeutic treatment option. Preparation varies by clinic; there is no Health Canada approved product in this category.
- Combining HA and steroids for knee OA patients can provide significant improvement in pain outcomes and may provide a more rapid onset, and longer duration of action than either therapy alone. However, there are potential concerns regarding cartilage loss with regular injecting this combination of therapies. There is insufficient evidence to support other combinations of intra-articular injection therapy.
Only some clinicians are trained in accurate injection delivery. “The accuracy of intra-articular injections depends on the joint and on the skills of the practitioner, imaging may improve accuracy.”
Steroid, HA and PRP injections can also be given with or without local anaesthetic. The effects of local anaesthesia on the cartilage are an emerging body of evidence as well.
